You’ve probably experienced foul smells before, but as bad as you remember them stinking, scientists say it’s nothing compared to the stench of thioacetone, the world’s smelliest chemical compound.
Trash left in the can too long on a summer day, sewage, or the smell of public toilets uncleaned for multiple days, we can all agree that they stink, but their stench is not classified as dangerous to human life. However the foul aroma of the rare chemical known as thioacetone, is. This simple molecule is somewhat tricky to produce, as at temperatures above -20°C it will clump together to form a solid called trithioacetone, but on the rare occasions that humanity did create it, thioacetone managed to make its presence felt, quite literally.
The most famous story about the stench of thioacetone dates back to1889, when workers at a factory in the German city of Freiberg attempted to produce the simple yet potent chemical, and accidentally created panic among the city’s residents. Their success led to “an offensive smell spreading rapidly over a great area of the town, causing fainting, vomiting and a panic evacuation”.

Photo: Jesse Bridgewater/Pixabay
Something similar happened in 1967, when British researchers Victor Burnop and Kenneth Latham attempted to use thioketones to create new polymers, and left a bottle of residue open for just a few moments. The accident caused nausea in people working in buildings hundreds of yards away.
“Recently we found ourselves with an odor problem beyond our worst expectations,” researchers at the Eso Research station reported. “During early experiments, a stopper jumped from a bottle of residues, and, although replaced at once, resulted in an immediate complaint of nausea and sickness from colleagues working in a building two hundred yards [180 m] away. Two of our chemists who had done no more than investigate the cracking of minute amounts of trithioacetone found themselves the object of hostile stares in a restaurant and suffered the humiliation of having a waitress spray the area around them with a deodorant.”
In the early 1960s, when Professor Roland Mayer at Dresden University of Technology, embarked on a quest to a group of aromatic sulfur-containing chemical compounds called thioketones, he was aware of the foul reputation of thioacetone, but he was still surprised by the stench of it, writing “the smell of this unstable red oil is indeed almost indescribable”.
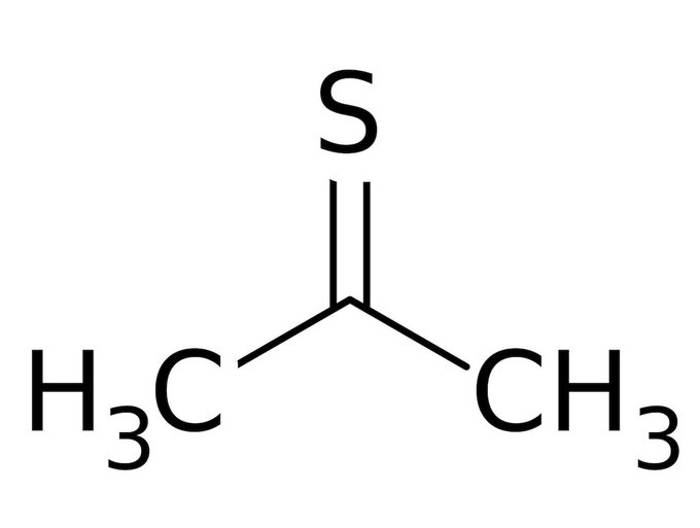
Interestingly, thioacetone is not that complicated of a chemical, but no one seems to know exactly why it smells as bad as it does. The sulfur attached to its chemical structure is more than likely the culprit, but why this particular compound smells so much worse than others remains an enigma, one that not many chemists are eager to solve.
“It merely stinks. But it does so relentlessly and unbearably,” “medicinal chemist Derek Lowe told Sciencemag. “It makes innocent downwind pedestrians stagger, clutch their stomachs, and flee in terror. It reeks to a degree that makes people suspect evil supernatural forces.”
According to Benjamin Andrikopoulos, a chemistry masters student at the University of Melbourne, the potency of thioacetone may have something to do with our evolution. Since times immemorial, humans have associated sulfur with rotting foods, with those who could more easily detect the smell avoiding illness or death, and passing on the strong sense of smell to their offspring.
Over tens of thousands of years, we have become able to smell sulfur-containing substances in amounts of 1 part per billion or even 1 part per trillion, the equivalent of half a teaspoon of sugar in an Olympic-sized swimming pool of water. With thioacetone, our sense of smell is even more impressive, at least according to the reports of researchers who participated in the 1967 Eso Research Station experiment.
“The odors defied the expected effects of dilution since workers in the laboratory did not find the odors intolerable … and genuinely denied responsibility since they were working in closed systems,” they wrote. “To convince them otherwise, they were dispersed with other observers around the laboratory, at distances up to a quarter of a mile [0.40 km], and one drop of either acetone gem-dithiol or the mother liquors from crude trithioacetone crystallisations were placed on a watch glass in a fume cupboard. The odor was detected downwind in seconds.”
To make matters worse, the smell of thioacetone is ‘sticky’, as well, meaning it can take days for even the smallest trace of smell to disappear. As curious as I am about the legendary potency of this compound, I hope I never get to smell it for myself. I though seafood sauce left to rot for decades smelled bad, but this sounds even worse.






